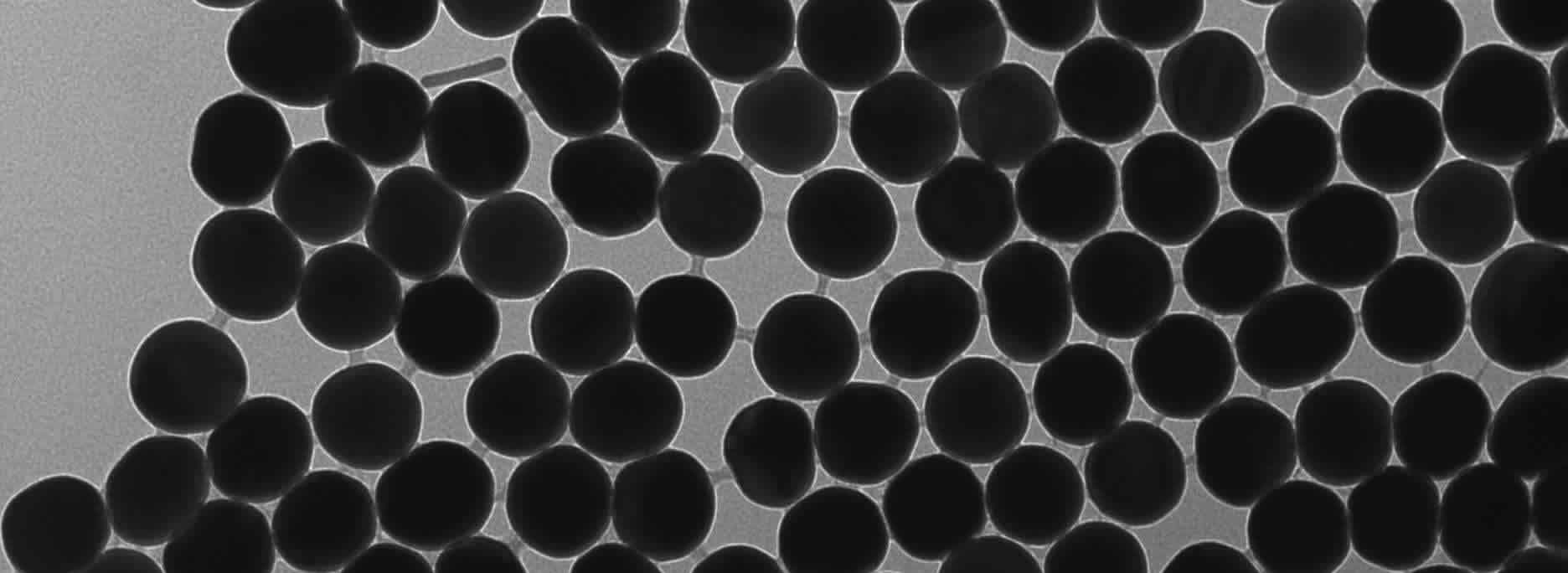
Why Do Different Sized Gold Nanoparticles Have Different SPRs?
Gold nanoparticles are widely studied for their unique optical properties, notably their ability to exhibit Surface Plasmon Resonance (SPR). SPR occurs when conduction electrons on the nanoparticle surface oscillate in resonance with incident light. This resonance is highly sensitive to the size, shape, and environment of the nanoparticle. In this article, we explain why different sized gold nanoparticles exhibit different SPRs.
The Basics of Surface Plasmon Resonance
Surface Plasmon Resonance refers to the collective oscillation of electrons in response to light. This phenomenon enhances the absorption and scattering of light at particular wavelengths, giving gold nanoparticles their distinctive colors.
When the electrons on the surface of gold nanoparticles resonate with light, this creates strong absorption peaks. The wavelength at which this absorption occurs is known as the SPR peak. This peak shifts depending on several factors, the most significant being the nanoparticle size.
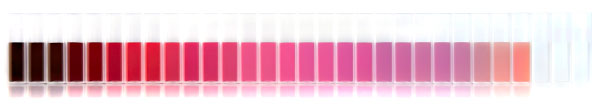
An image showing the color change of spherical gold nanoparticles as they increase in size from 1.8nm (left) to 1500nm (right). The light is setup so that you see both the absorption and scattering.
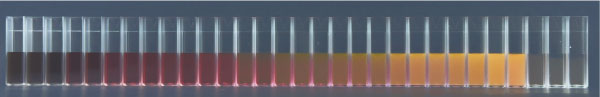
An image showing the color change of spherical gold nanoparticles as they increase in size from 1.8nm (left) to 1500nm (right). The light is setup so that you see only scattering.
The Effect of Size on SPR
-
Smaller Nanoparticles (1-20 nm):
- SPR peak in the visible region: Small gold nanoparticles tend to have SPR peaks around 520-530 nm, which corresponds to green light.
- Reason: In smaller nanoparticles, the electron cloud moves more freely, requiring less energy to reach resonance, which results in absorption in the visible spectrum.
- Larger Nanoparticles (Above 50 nm):
- SPR peak shifts to red/infrared: As gold nanoparticles grow larger, the SPR peak shifts toward longer wavelengths (red and infrared). This is because the larger the nanoparticle, the more the electron oscillation is dampened, requiring higher energy (longer wavelengths) to resonate.
- Reason: Larger nanoparticles have more complex oscillation patterns and increased surface scattering, leading to red-shifted SPR.
Shape and Environment
- Shape: Non-spherical shapes, such as rods or stars, show multiple SPR peaks because different parts of the nanoparticle interact with light differently. For example, gold nanorods can have transverse and longitudinal SPR modes.
- Environment: The dielectric constant of the surrounding medium also affects the SPR. A higher refractive index in the surrounding medium can shift the SPR peak to longer wavelengths.
Applications of SPR in Gold Nanoparticles
Different sized gold nanoparticles with specific SPR peaks are used in a variety of applications, such as:
- Biosensing: Changes in SPR can be detected when molecules bind to the nanoparticle surface, making gold nanoparticles ideal for sensors.
- Medical Imaging: Gold nanoparticles of specific sizes are used in imaging techniques, leveraging their enhanced scattering properties.
- Photothermal Therapy: The SPR effect is used to generate heat for targeted destruction of cancer cells.
For more information and to explore their products, visit the Nanopartz™ website. Happy researching!
Go here for Nanopartz Gold Nanoparticles