Intrinsic SERS - Nanopartz™
Intrinsic SERS (iSERS) utilizes Nanopartz Accurate Spherical Gold Nanoparticles and Gold Nanorods to enhance the Raman signals of analytes in solutions and solvents. Nanopartz tailer fit their gold nanoparticles for your application. Their SERS nanoparticles are designed to optimize Raman scattering cross sections with laser excitations 500nm to 2microns utilizing Spherical Gold and Gold Nanorods. Surface preparations can withstand user solutions of highly saline solutions including PBS, to organic solvent solutions as Toluene.
This product comes highly concentrated in 1mL vials, ready for dilution of the solvent of your choice.
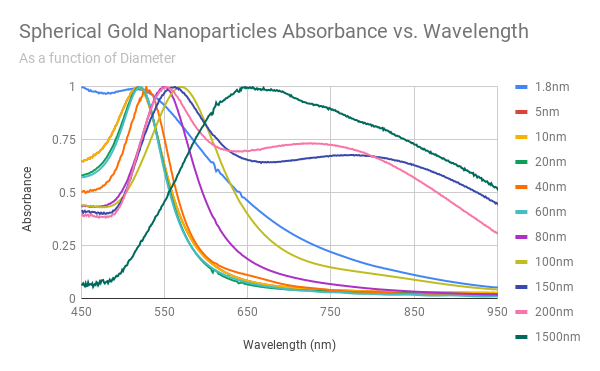
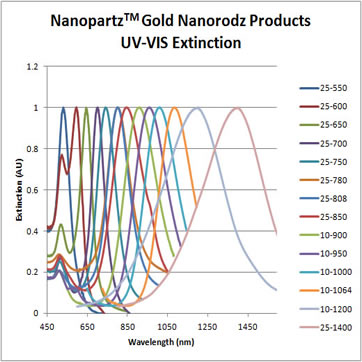
Every batch is completely characterized including size, monodispersity, aggregation, residual chemicals, and concentration. A Certificate of Analysis (COA) is provided for every order exhibiting TEM and UV-VIS images and data, as well as DLS data.

Nanopartz Intrinsic SERS Nanoparticles can work in a wide variety of solutions and solvent
Solution/Solvent |
iSERS |
| DI Water | Yes |
| PBS | Yes |
| Saline | Yes |
| Effluent | Yes |
| Serum | Yes |
Ethanol |
Yes |
Methanol |
Yes |
Isopropanol |
Yes |
Acetone |
Yes |
DMSO |
Yes |
DMF |
Yes |
Acetonitrile |
Yes |
Toluene |
Yes |
Part # |
Diameter (nm) |
Peak SPR Wavelength (nm) |
NPS/ml |
Molarity (pM) |
Moles |
Molar Ext. (M-1cm-1) |
Absorption Molar Ext. (M-1cm-1) |
Scattering Molar Ext. (M-1cm-1) |
Size Dispersity %PDI |
Size Accuracy (+/- nm) |
PIS11-1.8 |
1.8 |
500 |
4.25E+16 |
7.10E+07 |
7.09E-08 |
7.05E+05 |
7.05E+05 |
0.00E+00 |
<35% |
0.1 |
PIS11-2.2 |
2.2 |
502 |
2.33E+16 |
3.90E+07 |
3.88E-08 |
1.29E+06 |
1.29E+06 |
0.00E+00 |
<25% |
0.1 |
PIS11-3 |
3 |
506 |
9.19E+15 |
1.50E+07 |
1.53E-08 |
3.26E+06 |
3.26E+06 |
0.00E+00 |
<20% |
0.1 |
PIS11-4 |
4 |
510 |
3.88E+15 |
6.50E+06 |
6.46E-09 |
7.74E+06 |
7.74E+06 |
0.00E+00 |
<20% |
1 |
PIS11-5 |
5 |
512 |
1.99E+15 |
3.30E+06 |
3.31E-09 |
1.51E+07 |
1.51E+07 |
0.00E+00 |
<20% |
2 |
PIS11-10 |
10 |
516 |
2.48E+14 |
4.10E+05 |
4.14E-10 |
1.21E+08 |
1.21E+08 |
0.00E+00 |
<15% |
2 |
PIS11-15 |
15 |
518 |
7.35E+13 |
1.20E+05 |
1.23E-10 |
4.08E+08 |
4.08E+08 |
0.00E+00 |
<15% |
2 |
PIS11-20 |
20 |
520 |
3.10E+13 |
5.20E+04 |
5.17E-11 |
9.67E+08 |
9.67E+08 |
0.00E+00 |
<10% |
2 |
PIS11-25 |
25 |
521 |
1.59E+13 |
2.60E+04 |
2.65E-11 |
1.89E+09 |
1.89E+09 |
0.00E+00 |
<10% |
2 |
PIS11-30 |
30 |
523 |
9.19E+12 |
1.50E+04 |
1.53E-11 |
3.26E+09 |
3.26E+09 |
0.00E+00 |
<6% |
2 |
PIS11-35 |
35 |
526 |
5.79E+12 |
9.60E+03 |
9.65E-12 |
5.18E+09 |
5.18E+09 |
0.00E+00 |
<6% |
2 |
PIS11-40 |
40 |
527 |
3.88E+12 |
6.50E+03 |
6.46E-12 |
7.74E+09 |
7.72E+09 |
2.24E+07 |
<4% |
2 |
PIS11-45 |
45 |
529 |
2.72E+12 |
4.50E+03 |
4.54E-12 |
1.10E+10 |
1.08E+10 |
2.03E+08 |
<4% |
2 |
PIS11-50 |
50 |
531 |
1.99E+12 |
3.30E+03 |
3.31E-12 |
1.51E+10 |
1.45E+10 |
5.88E+08 |
<4% |
2 |
PIS11-55 |
55 |
533 |
1.49E+12 |
2.50E+03 |
2.49E-12 |
2.01E+10 |
1.88E+10 |
1.30E+09 |
<4% |
2 |
PIS11-60 |
60 |
536 |
1.15E+12 |
1.90E+03 |
1.91E-12 |
2.61E+10 |
2.36E+10 |
2.48E+09 |
<4% |
2 |
PIS11-65 |
65 |
539 |
9.04E+11 |
1.50E+03 |
1.51E-12 |
3.32E+10 |
2.89E+10 |
4.33E+09 |
<4% |
2 |
PIS11-70 |
70 |
542 |
7.23E+11 |
1.20E+03 |
1.21E-12 |
4.15E+10 |
3.44E+10 |
7.09E+09 |
<4% |
2 |
PIS11-75 |
75 |
545 |
5.88E+11 |
9.80E+02 |
9.80E-13 |
5.10E+10 |
4.00E+10 |
1.10E+10 |
<4% |
2 |
PIS11-80 |
80 |
549 |
4.85E+11 |
8.10E+02 |
8.08E-13 |
6.19E+10 |
4.54E+10 |
1.65E+10 |
<4% |
2 |
PIS11-85 |
85 |
553 |
4.04E+11 |
6.70E+02 |
6.73E-13 |
7.42E+10 |
5.03E+10 |
2.39E+10 |
<4% |
2 |
PIS11-90 |
90 |
558 |
3.40E+11 |
5.70E+02 |
5.67E-13 |
8.81E+10 |
5.44E+10 |
3.37E+10 |
<4% |
2 |
PIS11-95 |
95 |
563 |
2.89E+11 |
4.80E+02 |
4.82E-13 |
1.04E+11 |
5.72E+10 |
4.65E+10 |
<4% |
2 |
PIS11-100 |
100 |
569 |
2.48E+11 |
4.10E+02 |
4.14E-13 |
1.21E+11 |
5.82E+10 |
6.27E+10 |
<4% |
2 |
PIS11-150 |
150 |
612 |
7.35E+10 |
1.20E+02 |
1.23E-13 |
4.08E+11 |
0.00E+00 |
4.08E+11 |
<4% |
10 |
%PDI = Std Dev/Size
|
See Tech Note TN801 for definition of terms and method of analysis
|
All specs typical. May vary batch to batch. Exact values are measured for each batch
|
Shape monodispersity (% spheres) > 99.9%
|
Residual Chemicals < 0.1%
|
Part # |
Diameter (nm) |
Length (nm) |
Peak SPR Wave (nm) |
Aspect Ratio |
OD SPR (AU) |
Peak LSPR Wave (nm) |
OD LSPR (AU) |
NPS /mL |
Wt. conc (ug/ml) |
Wt. % |
PPM |
Molarity (nM) |
Picomoles |
SPR Molar Ext. (M-1cm-1) |
LSPR Molar Ext. (M-1cm-1) |
Peak SPR (nm) |
SPR Linewidth 80% (nm) |
PIS-2100 |
10 |
175 |
2100 |
17.5 |
50 |
510 |
10 |
6.73E+12 |
1750 |
0.18% |
1750 |
11.21 |
1.12E+01 |
4.46E+09 |
8.92E+08 |
1900-2300 |
300 |
PIS-1400 |
25 |
245 |
1400 |
9.8 |
50 |
514 |
15 |
1.12E+12 |
2500 |
0.25% |
2500 |
1.86 |
1.86E+00 |
2.69E+10 |
8.06E+09 |
1232-1500 |
150 |
PIS-1064 |
25 |
137 |
1064 |
5.5 |
50 |
514 |
15 |
2.05E+12 |
2500 |
0.25% |
2500 |
3.42 |
3.42E+00 |
1.46E+10 |
4.38E+09 |
1022-1232 |
150 |
PIS-980 |
25 |
119 |
980 |
4.8 |
50 |
514 |
15 |
2.39E+12 |
2500 |
0.25% |
2500 |
3.98 |
3.98E+00 |
1.26E+10 |
3.77E+09 |
965-1022 |
150 |
PIS-950 |
25 |
102 |
950 |
4.1 |
50 |
514 |
15 |
2.82E+12 |
2500 |
0.25% |
2500 |
4.7 |
4.70E+00 |
1.06E+10 |
3.19E+09 |
925-965 |
150 |
PIS-900 |
25 |
96 |
900 |
3.8 |
50 |
514 |
15 |
3.01E+12 |
2500 |
0.25% |
2500 |
5.02 |
5.02E+00 |
9.95E+09 |
2.99E+09 |
875-925 |
150 |
E12-25-850 |
25 |
93 |
850 |
3.7 |
50 |
514 |
15 |
3.12E+12 |
2500 |
0.25% |
2500 |
5.2 |
5.20E+00 |
9.61E+09 |
2.88E+09 |
829-875 |
150 |
E12-25-808 |
25 |
90 |
808 |
3.6 |
50 |
514 |
15 |
3.24E+12 |
2500 |
0.25% |
2500 |
5.39 |
5.39E+00 |
9.27E+09 |
2.78E+09 |
794-829 |
150 |
E12-25-780 |
25 |
87 |
780 |
3.5 |
50 |
514 |
15 |
3.36E+12 |
2500 |
0.25% |
2500 |
5.6 |
5.60E+00 |
8.93E+09 |
2.68E+09 |
765-794 |
150 |
E12-25-750 |
25 |
85 |
750 |
3.4 |
50 |
514 |
15 |
3.45E+12 |
2500 |
0.25% |
2500 |
5.75 |
5.75E+00 |
8.70E+09 |
2.61E+09 |
725-765 |
65 |
E12-25-700 |
25 |
75 |
700 |
3 |
50 |
514 |
15 |
3.96E+12 |
2500 |
0.25% |
2500 |
6.61 |
6.61E+00 |
7.57E+09 |
2.27E+09 |
675-725 |
60 |
E12-25-650 |
25 |
71 |
650 |
2.8 |
50 |
514 |
15 |
4.22E+12 |
2500 |
0.25% |
2500 |
7.03 |
7.03E+00 |
7.11E+09 |
2.13E+09 |
625-675 |
60 |
E12-25-600 |
25 |
57 |
600 |
2.3 |
50 |
514 |
15 |
5.43E+12 |
2500 |
0.25% |
2500 |
9.05 |
9.05E+00 |
5.52E+09 |
1.66E+09 |
575-625 |
60 |
E12-25-550 |
25 |
34 |
550 |
1.4 |
50 |
514 |
15 |
1.01E+13 |
2500 |
0.25% |
2500 |
16.91 |
1.69E+01 |
2.96E+09 |
8.87E+08 |
525-575 |
60 |
E12-40-1064 |
40 |
208 |
850 |
5.2 |
50 |
520 |
20 |
5.30E+11 |
2500 |
0.25% |
2500 |
0.88 |
8.84E-01 |
5.66E+10 |
2.26E+10 |
1022-1232 |
150 |
E12-40-980 |
40 |
180 |
850 |
4.5 |
50 |
520 |
20 |
6.19E+11 |
2500 |
0.25% |
2500 |
1.03 |
1.03E+00 |
4.84E+10 |
1.94E+10 |
875-1022 |
150 |
E12-40-850 |
40 |
148 |
850 |
3.7 |
50 |
520 |
20 |
7.67E+11 |
2500 |
0.25% |
2500 |
1.28 |
1.28E+00 |
3.91E+10 |
1.57E+10 |
829-875 |
150 |
E12-40-808 |
40 |
134 |
808 |
3.4 |
50 |
520 |
20 |
8.56E+11 |
2500 |
0.25% |
2500 |
1.43 |
1.43E+00 |
3.51E+10 |
1.40E+10 |
794-829 |
150 |
E12-40-780 |
40 |
124 |
780 |
3.1 |
50 |
520 |
20 |
9.33E+11 |
2500 |
0.25% |
2500 |
1.55 |
1.55E+00 |
3.22E+10 |
1.29E+10 |
765-794 |
150 |
E12-40-750 |
40 |
112 |
750 |
2.8 |
50 |
520 |
20 |
1.05E+12 |
2500 |
0.25% |
2500 |
1.74 |
1.74E+00 |
2.87E+10 |
1.15E+10 |
725-765 |
150 |
E12-40-700 |
40 |
92 |
700 |
2.3 |
50 |
520 |
20 |
1.31E+12 |
2500 |
0.25% |
2500 |
2.19 |
2.19E+00 |
2.29E+10 |
9.14E+09 |
675-725 |
60 |
E12-40-650 |
40 |
80 |
650 |
2 |
50 |
520 |
20 |
1.55E+12 |
2500 |
0.25% |
2500 |
2.58 |
2.58E+00 |
1.94E+10 |
7.75E+09 |
625-675 |
60 |
E12-40-600 |
40 |
68 |
600 |
1.7 |
50 |
520 |
20 |
1.89E+12 |
2500 |
0.25% |
2500 |
3.15 |
3.15E+00 |
1.59E+10 |
6.35E+09 |
575-625 |
60 |
E12-40-550 |
40 |
60 |
550 |
1.5 |
50 |
520 |
20 |
2.21E+12 |
2500 |
0.25% |
2500 |
3.69 |
3.69E+00 |
1.36E+10 |
5.42E+09 |
525-575 |
60 |
E12-50-1064 |
50 |
245 |
1064 |
4.9 |
50 |
530 |
25 |
2.89E+11 |
2500 |
0.25% |
2500 |
0.48 |
4.82E-01 |
1.04E+11 |
5.18E+10 |
980-1232 |
150 |
E12-50-808 |
50 |
145 |
808 |
2.9 |
50 |
530 |
25 |
5.15E+11 |
2500 |
0.25% |
2500 |
0.86 |
8.58E-01 |
5.83E+10 |
2.91E+10 |
750-850 |
150 |
E12-50-700 |
50 |
110 |
700 |
2.2 |
50 |
530 |
25 |
7.08E+11 |
2500 |
0.25% |
2500 |
1.18 |
1.18E+00 |
4.24E+10 |
2.12E+10 |
650-750 |
150 |
E12-50-600 |
50 |
100 |
600 |
2 |
50 |
530 |
25 |
7.93E+11 |
2500 |
0.25% |
2500 |
1.32 |
1.32E+00 |
3.78E+10 |
1.89E+10 |
550-650 |
150 |
E12-70-750 |
70 |
120 |
750 |
1.7 |
50 |
530 |
25 |
3.49E+11 |
2500 |
0.25% |
2500 |
0.58 |
5.81E-01 |
8.60E+10 |
4.30E+10 |
700-800 |
150 |
E12-70-650 |
70 |
105 |
650 |
1.5 |
50 |
530 |
25 |
4.13E+11 |
2500 |
0.25% |
2500 |
0.69 |
6.88E-01 |
7.27E+10 |
3.63E+10 |
600-700 |
150 |
E12-70-550 |
70 |
91 |
550 |
1.3 |
50 |
530 |
25 |
4.98E+11 |
2500 |
0.25% |
2500 |
0.83 |
8.30E-01 |
6.02E+10 |
3.01E+10 |
500-600 |
150 |
Optical Density >= 200, volume = 0.25mL
|
Specs shown in table are for dilution at 1mL
|
Solution default is 18MEG DI water
|
Residual Chemicals < 0.1%
|
Part # |
Diameter (nm) |
Length (nm) |
Aspect Ratio |
Peak SPR Wave (nm) |
SPR OD (1cm) |
Microgold /mL |
moles |
Wt. conc (ug/ml) |
Wt. % |
PPM |
Molarity (pM) |
SPR Molar Ext. (M-1cm-1) |
E13-500 |
75 |
500 |
6.7 |
510 |
0.25 |
6.18E+09 |
1.03E-14 |
250 |
0.03% |
250 |
10.3 |
2.43E+07 |
E13-1000 |
100 |
1000 |
10 |
510 |
0.25 |
1.71E+09 |
2.85E-15 |
250 |
0.03% |
250 |
2.85 |
8.78E+07 |
| Specs are for product diluted to 1mL, product comes 0.25mg in 0.25mL |
SPR = Transverse SPR peak
|
Longitudinal has no SPR peak
|
Shape monodispersity (% rods) > 95%
|
Size variation +/-10% (both dimensions)
|
Part # |
Diameter (nm) |
Length (nm) |
Aspect Ratio |
Peak SPR Wave (nm) |
Nanowires Vol (nm3) |
Nanowires /mL |
Wt. conc (ug/ml) |
Wt. % |
PPM |
Molarity (pM) |
SPR Molar Ext. (M-1cm-1) |
E14-1000 |
75 |
1000 |
13 |
510 |
4.31E+06 |
3.01E+09 |
250 |
0.03% |
250 |
5.02 |
9.96E+06 |
E14-2000 |
75 |
2000 |
27 |
510 |
8.72E+06 |
1.49E+09 |
250 |
0.03% |
250 |
2.48 |
2.02E+07 |
E14-4000 |
75 |
4000 |
53 |
510 |
1.76E+07 |
7.39E+08 |
250 |
0.03% |
250 |
1.23 |
4.06E+07 |
E14-6000 |
75 |
6000 |
80 |
510 |
2.64E+07 |
4.91E+08 |
250 |
0.03% |
250 |
0.82 |
6.10E+07 |
E14-10000 |
75 |
10000 |
133 |
510 |
4.40E+07 |
2.94E+08 |
250 |
0.03% |
250 |
0.49 |
1.02E+08 |
E14-20000 |
100 |
20000 |
200 |
510 |
1.57E+08 |
8.27E+07 |
250 |
0.03% |
250 |
0.14 |
3.63E+08 |
E14-40000 |
140 |
40000 |
286 |
510 |
6.15E+08 |
2.11E+07 |
250 |
0.03% |
250 |
0.04 |
1.42E+09 |
| Specs are for product diluted to 1mL, product comes 0.25mg in 0.25mL |
SPR = Transverse SPR peak
|
Longitudinal has no SPR peak
|
Shape monodispersity (% rods) > 95%
|
Size variation +/-10% (both dimensions)
|
Organic Nanorod with Nanopartz™ covalently bonded polymer
Solvent |
PVP |
Polystyrene |
Nanopartz Organic |
Nanopartz Conductive |
| Covalent? | No | Yes | Yes | No |
Ethanol |
Yes |
Yes |
Yes |
Yes |
Methanol |
Yes |
Yes |
Yes |
Yes |
Isopropanol |
Yes |
Yes |
Yes |
Yes |
Acetone |
Yes |
Yes |
Yes |
Yes |
DMSO |
Yes |
Yes |
Yes |
Yes |
DMF |
Yes |
Yes |
Yes |
Yes |
Acetonitrile |
No |
Yes |
Yes |
Yes |
Toluene |
No |
Yes |
Yes |
No |
Chloroform |
No |
No |
Yes |
No |
Silicon Oil |
No |
No |
Yes |
No |
Hexane |
No |
No |
Yes |
No |
Heptane |
No |
No |
Yes |
No |
Decanol |
No |
No |
Yes |
No |
Composition
These nanoparticles are shipped in solvent of choice with no measurable residual reactants.
Custom Formulation
Please contact us.
Quantity
This product is available in 0.25mL volumes and larger. This product is expected to be diluted.
Introductory Kits
Please contact us.
Delivery
Standard sizes are in stock. Special order sizes are shipped in two weeks or less. All domestic shipments are sent Fed Ex Standard Overnight delivery, international Fed Ex Priority 2 day. No shipments on Fridays except for dried particles. Saturday shipping available for extra charge.
Functionalization
This product comes with a number of different covalent and adsorbed options. For adsorbed option, please choose PVP.
Shelf Life/Storage Temperature
This product is guaranteed for six months and should be stored at 4 °C after opening. Care must be taken to only use sterile glassware when working with this product.
Toxicity
This product is known to be noncytotoxic (other than the solvent).
Certifications
Every order comes with a Certification of Analysis that includes the following information. We use calibration traceable:
UV-VIS (Agilent 8453) for extinction and concentration measurements
NIR (Cary 500) for NIR extinction and concentration measurements
DLS (Malvern Nano ZS) for zeta potential measurement
ICP-MS (Varian 820-MS) for gold mass measurements
TEM (Phillips CM-100 100KV) for sizing
TN801 UV VIS to Determine Size and Concentration
Note: SDS are based on solvent. Please request an SDS if for your solvent
How much thickness does your proprietary capping agent add?
Anywhere from 1-5nm.
How do I determine the concentration of the product?
Simply divide the number of nanoparticles (nps) found on the included COA by the amount of solution you add.
What is a metamaterial?
This is where the concentration is so high, the product acts like mercury.
What can I resuspend the metamaterial into?
Any solvent!
Can I resuspend in water?
No
What is the shelf life if I never open the package and keep refrigerated?
Many years.
Should I store in the refrigerator?
Yes.
How do you size your gold nanoparticles? Does the size include the capping agent?
We use three methods to specify our gold nanoparticles; TEM, UV VIS, and DLS. Each has its own advantages and disadvantages, and we use a weighted system to take advantage of each methods strengths. In the end, we place the strongest weight to the TEM method, particularly since we use samples sizes greater than 50 particles for each lot.
Do you really provide a TEM for my specific lot?
Yes, and not just for 5-10 particles, rather 50-100 are standard.
What is PDI?
PDI refers to polydispersity index and is equal to the standard deviation of the particle sizes divided by the average size.
What if I need to know the polymer being used?
Choose Polystyrene.
What if I need to a non covalent polymer so I can do further conjugations?
Choose PVP.
"I have been using the Nanopartz Organic Spherical Gold Nanoparticles in my research and they have worked extremely well due to the moderate hydrophilicity and high concentration. I highly recommend this product."
Mingmeng Zhang
PhD Student
Arizona State University
Optical nonlinearities and ordering in aqueous and organic colloidal plasmonic materials
PB Alvaro Jr - 2018 -
… 36 Comparing the Aqueous Solution with the Organic Solution ..... 39 … 67
V111 Page 9. LIST OF TABLES Table Page Table 3.2.1. Characteristics
of our gold nanorod aqueous solutions from NanoPartz …
Electric field induced orientational order of gold nanorods in dilute organic suspensions
J Fontana, GKB da Costa, JM Pereira… - Applied Physics …, 2016 -
… Electric field induced orientational order of gold nanorods in dilute organic suspensions …
Toxicity of organic and inorganic nanoparticles to four species of white-rot fungi
TPS Galindo, R Pereira, AC Freitas… - Science of the total …, 2013 - Elsevier
… Three inorganic (Fe/Co, CdSe/ZnS-QD, gold nanorods–NP-Au) and two organic (vesicles of
sodium dodecyl sulfate and … Luis, MO, USA), Nanopartz™ (Salt Lake City, UT, USA), Akzo Nobel
Surface ChemistryAB (Stenungsund, Sweden), Sigma-Aldrich, Danisco Ingredients …
Enhanced vortex pinning in Nb using proximity effect through organic molecules
E Katzir, N Sukenik, H Alpern, S Yochelis… - Journal of Physics …, 2018
… (a) Arrays of Au or Ni islands evaporated using standard e-beam lithography. (b) Au NPs attached
via organic molecules in selective regions … The solution consisted of 3.75 Wt% gold NPs attached
to MPS and dispersed in ethanol (purchased from Nanopartz, Inc …
Increasing the Transition Temperature of High-T C Superconductor Thin Films by Organic Linking of Gold Nanoparticles
H Alpern, M Periyasamy, J Tannous, G Jung… - … of Superconductivity and …, 2020 - Springer
… of two optimally doped cuprates, YBa2Cu3O7-δ (YBCO) and La1.85Sr0.15CuO4, (LSCO) increase
upon linking Au NPs to their surface via organic mole- cules … of 10 μL of 1.25 × 10 −2 wt% 10 nm
Au NPs linked to MPS molecules stabilized in ethanol (Nanopartz, Germany), also …
These particles utilize a NPO - a Nanopartz™ formulated coating - that shields the particle from the effects of abrasive solvents, and yet, still provide the optical properties inherent to the particles. When shipped as a metamaterial form, can be resuspended in any solvent.
Applications
Sensors
Solar Cells
Flexible Optical Polarizers and Filters
Spin coating
Negative refractive index materials
Liquid Crystals
Security anti-forge materials
| Nanopartz™ in vitro Gold Nanoparticles | PEG Gold Nanoparticles | |
| Stability (salt,pH,chemicals) | High | Medium |
| Monovalent | Yes | No |
| Nonspecific binding | Very low | Medium |
| Sterilization | Yes | No |
- By choosing the NPO Nanopartz Organic Polymer as a metamaterial, you have chosen a product that can be resuspended in any solvent
- These products work in a number of different solvents including toluene, chloroform, and more.
Example part number is E11-10-NPO-MM-50-0.25 where:
E11 - Product family number for Organic Spherical Gold Nanoparticles. For Organic Gold Nanorods, its E12 and so forth.
10 - Sphere diameter in nanometers. Other choices are 1.8 to 1500
NPO - Nanopartz Organic Polymer. Other coatings are Polystyrene, PVP, and NPV, Nanopartz Conductive Coating
MM - Metamaterial. Other choices are ETOH, Toluen, and more.
50 - Optical Density, in this case OD=50 at 1mL
0.25 - Volume (mL). Other choices are 1mL, and more.
Ordering by scrolling down and selecting the options from the selection below.