Customer Spotlight - Gold Nanoparticle Mediated Multi-Modal CT Imaging of Hsp70 Membrane-Positive Tumors
For the first time, scientists from the Technical University of Munich have successfully utilized membrane-Hsp70 functionalized Gold-Nanoparticles for tumor-specific spectral CT imaging in preclinical settings. As the central element of this study, PEG-amine-coated gold nanoparticles from Nanopartz were used, which were further functionalized with anti-membrane Hsp70 antibodies, rendering them highly tumor-specific for in vivo applications.
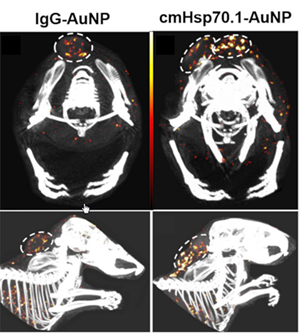
Tumor detection using spectral-CT. Upper Row: axial view, bottom row: sagital view. AuNP amounts are pseudo-coloured from black (6.5 mg/mL) over red (10 mg/mL) to white (13.5 mg/mL). (Left Column) spectral CT-views of mice injected with IgG-AuNPs; (Right Column) spectral CT-views of mice injected with cmHsp70.1-AuNPs |
Heat shock protein 70 as tumor target
In addition to the physiological, cytosolic expression in all nucleated cells, the molecular chaperone Heat shock protein 70 (Hsp70, Hsp70-1, HspA1A, #3303) is exclusively presented on the plasma membrane of malignantly transformed cells. Screening of tumor biopsies of over 1200 patients has shown that the majority of primarily diagnosed malignancies of multiple entities, but none of the tested corresponding normal tissues, exhibited a membrane Hsp70-positive phenotype. Evidence suggest that, throughout the evolution of the mammalian immune system, Hsp70 specific receptors have been developed as a first line of defense against cancerous diseases. Bionic adaptation of this finding enabled cutting-edge research to render more precisely diagnostic and therapeutic tumor-targeting approaches.
In the scientific journal "Cancers" (https://www.mdpi.com/2072-6694/12/5/1331), German scientists showed for the first time that gold nanoparticles can be rendered tumor specific under utilization of an anti-Hsp70 antibody, named cmHsp70.1, providing a powerful contrast agent for spectral CT applications. In preclinical trials, significant amounts of Hsp70-activated nanoparticles were found enriched in the tumors, following intravenous application. The Hsp70 mAb-conjugated gold nanoparticles were developed by the scientists around Dr. Stefan Stangl and Prof. Gabriele Multhoff, who holds the Chair of "Experimental Radiooncology and Radiation Therapy" at the medical faculty of the Technical University of Munich, in close cooperation with Dr. Melanie Kimm from the Department of Radiology of Klinikum rechts der Isar of the Technical University of Munich.
Molecular targeting of membrane Hsp70-positive tumors
Why are Hsp70-activated nanoparticles in the focus of tumor targeting?
“Plasmamembrane-associated Hsp70 provides the ideal target structure for diagnostic and therapeutic tumor targeting in vivo, as it is a tumor cell-exclusive phenomenon” explains Dr. Stangl, one of the initiators of the study. “By utilizing it as a target structure, we can direct compounds of interest to home into the tumor more precisely and at higher quantities than their traditional equivalents, while omitting the healthy body tissues. Furthermore, membrane-bound Hsp70 has been found in the vast majority of the cases of the multiple tumor entities studied by us and our colleagues around the world, making it a pan-tumor marker. In in vitro experiments, we observed a rapid internalization of membrane-associated Hsp70 into the cytoplasm, ensuring a strong intracellular accumulation of the targeting compounds - even those of larger sizes. This helps us to deliver sufficiently high doses to the focal point, enhancing the effectivity of treatments.
Here, gold-nanoparticles are of particular interest for our theranostic tumor targeting approaches. The combination of therapy and diagnosis with a single ´magic bullet´ is very appealing, but it requires multiple distinct features of the applied drug. Gold nanoparticles prove highly suitable for such projects, since on the one hand they serve as specific contrast agents for spectral CT imaging, excluding any potential false-positives. For cancer treatment on the other hand, they display their therapeutic potential to intensify radiotherapy. Due to its high atomic number (Z = 79), gold emits a high number of secondary electrons upon irradiation. These so-called Auger-electrons are of high therapeutic efficacy, since they induce intense radiation-induced damage in the DNA backbone of cancer cells at low distances. The increased accumulation of the Hsp70-modified gold nanoparticles in close proximity to the nucleus was particularly noticeable to us. As a consequence, in the presence of gold nanoparticles, the therapeutic effect of the applied radiation is enhanced at the tumor side, helping us to protect the surrounding healthy tissue.” Dr. Stangl points out.
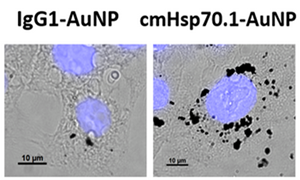
Uptake of AuNPs in tumor cells: Intracellular accumulation of IgG1-AuNPs (left), and cmHsp70.1-AuNPs (right) in 4T1 cells. |
Transition to the clinic
By establishing Hsp70-modified spherical gold nanoparticles as tumor-specific contrast agent, the group was facing the challenge to optimize the nanoparticles in a way that they exhibit a long blood circulation time, a rapid uptake of particles in the tumor cell, and avoid off-site targeting which could lead to side effects. "We know from our studies that Hsp70-binding Nanoparticles are rapidly internalized and detectable in the cytoplasm“ reports Dr. Stangl, "as the binding epitope is identical in mouse and human tumor cells, preclinical studies can likely quickly be transferred into the clinic.” Not only for external beam radiation therapy, but also for nano-brachytherapy, the approach of Dr. Kimm and Dr. Stangl represents an interesting target.
Spherical gold nanoparticles from Nanopartz play a crucial role
The centerpiece of our method are PEGylated spherical gold nanoparticles from Nanopartz which were in addition conjugated with amine residues“ explains Dr. Kimm. The amine residues were the anchers for the maleimide activation of the gold nanoparticles which finally were conjugated to sulfhydryl activated Hsp70 antibodies. In vivo applied Hsp70-modified gold nanoparticles were traced back predominantely in the tumor tissue using Spectral-CT imaging. No signs of toxicity were observed, but additional pharmacological and toxicological studies are needed to prove safety. In addition, a machine-learning approach was invented to improve accuracy of the histological analysis of Hsp70-gold nanoparticle biodistribution. In summary, Hsp70-modified gold nanoparticles are a promissing tool for radiotherapeutics as well as for CT-imaging. However, the feasibility of these specified gold nanoparticles as radiosensitizer and their usage in the clinic still needs more investigations.
Funding
This work was funded by DFG grants (SFB824/3, STA1520/1-1; ME 3718/5-1), Technische Universität München (TUM) within the DFG funding program Open Access Publishing. Additional financial support was provided by TaGoNaX DFG project №336532926, SFB824, and the Russian Foundation for Basic Research (RFBR) according to the research project №20-38-70039.
More information
Please contact Dr. Stefan Stangl, Central Institute for Translational Cancer Research (TranslaTUM), Klinikum rechts der Isar der Technischen Universität München, 81675 Munich, Germany, phone +49 89 4140 6013 (stefan.stangl@tum.de) or Dr. Melanie Kimm, Department of Diagnostic and Interventional Radiology, Klinikum rechts der Isar der Technischen Universität München, 81675 Munich, Germany (current address: Department of Radiology, University Hospital, LMU Munich, Marchioninistrasse 15, Munich, Germany. (melanie.kimm@med.uni-muenchen.de), or Dr. Melanie Kimm Professional Page and Klinikum rechts der Isar of the Technical University of Munich Website.
Kimm MA. et al. Gold Nanoparticle Mediated Multi-Modal CT Imaging of Hsp70 Membrane-Positive Tumors. Cancers (Basel) 12, 5 (2020). doi: 10.3390/cancers12051331. PMID: 32456049
Nanopartz Products Used for this Research
The products used for this research are our Accurate Spherical Gold Nanoparticles with a CTAB capping agent. CTAB is a micelle that provides a strong positive charge for the spherical gold. The product is found at Gold Nanoparticles.